Understanding the mechanisms by which genetic information is replicated is critical for basic biological organism knowledge as well as many useful applications in biomedical research and biotechnology. When a strand is being copied, one of the primary functions of a DNA polymerase enzyme is to help DNA recognize itself with high specificity.
What is DNA?
DNA stands for deoxyribose nucleic acid, a heredity material that contains instructions for protein synthesis. DNA is present in every cell of the living organism. In 1953 Watson and Crick determined the double helix structure of DNA. The structure of DNA mainly depends on Hydrogen bonding that stabilizes the structure of DNA.
What is hydrogen bonding?
Hydrogen bonding is an electrostatic force between a proton, generally Hydrogen and electronegative atoms that can be fluorine, Nitrogen, or oxygen. This bond is frailer than the covalent bond and ionic bond but more substantial than van der Waals forces. Hydrogen bonding is a particular type of dipole-dipole interaction between the molecules. Hydrogen bonds can be within a molecule’s atoms or between different molecule’s atoms. Hydrogen bonding can be between inorganic molecules such as in water and alcohol or within organic molecules such as DNA and proteins.
Generally, F, C, and O are covalently bond to Hydrogen. Due to F, N, and O atoms H atom gain a slight positive charge due to the high electronegativity. F, N, and O have an unshared pair of the electron due to which they gain negative charge. Mainly with the help of electrostatic forces, the donor atoms (C, O, or N) effectively share their hydrogen atom with the acceptor atom and form a hydrogen bond.
There are two conditions for Hydrogen bonding;
- There must be an electronegative atom in a molecule that will link to the hydrogen atom. The larger the electronegativity of the atom, the stronger the polarization.
- The electronegative atom’s size must be small because of the small size, and there must be a strong electrostatic force of attraction.
Types of hydrogen bonding:
There are two types of hydrogen bonding;
- Intermolecular hydrogen bonding
When hydrogen bonding takes place between different kinds of molecules of the same compound or between other compound’s molecules, the bonding is known as intermolecular hydrogen bonding, for example, hydrogen bonding in water or alcohol, etc.
- Intramolecular hydrogen bonding
When hydrogen bonding occurs within the molecule of a compound is known as intramolecular hydrogen bonding. In substances comprising two groups, it happens that one group includes a hydrogen atom connected to an electronegative atom, and the second group includes a strongly electronegative atom bonded to the other group’s weaker electronegative atom.
Hydrogen bonding in DNA:
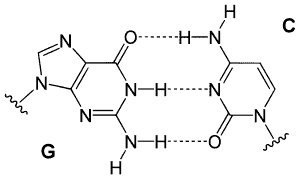
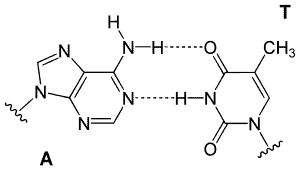
DNA is made up of four bases Adenine (A), Cytosine (C), Guanine (G), and Thymine (T). With the assistance of hydrogen bonding, the reciprocal base pairing of Guanine to Cytosine and Adenine to Thymine are correlated. These hydrogen bonds are what keep together the two ends of the DNA helix between complementary nucleotides. Each base can also form hydrogen bonds with the outside environment, such as water. The high strength of the billion hydrogen bonds in DNA allows them stable molecules, but these Externally and internally links of Hydrogen are limited. The hydrogen bonds on each nucleotide on the phosphate groups often combine to make the two DNA pieces fit into the molecular structure.
It is possible to clarify the pairing of DNA bases (a purine and a pyrimidine base). Hydrogen bonding between adjacent chains often divides the double helix and binds DNA chains together. The hydrogen bond among the bases, thus, enhances the hydrophobic effects that reinforce DNA. Within the helix, water repellent bases have been replaced, while polar exterior solvents enter the water. A fragile molecular force is hydrogen bonding, but it has an extra impact that reinforces DNA molecules. The bases are bonded with Hydrogen with an energy of 1 to 5 kcal/mol (4 to 21 kg/mol).
A comparable pattern is formed by hydrogen bonding in one purine’s DNA bases (guanine and adenine) and one pyrimidine (Cytosine and Thymine). Guanine and cytosine pairings are very close to adenine and thymine pairings. Three hydrogen bonds hold Cytosine and guanine together. Two hydrogen bonds share the adenine and thymine combination, rendering the connection marginally weaker and broader.
Hydrogen bonds are weak, noncovalent interactions, but in a DNA double helix, the vast number of hydrogen bonds between complementary base pairs combine to provide the system with considerable stability.
Hydrogen bonding in the functioning of DNA:
Watson crick-type hydrogen base-pair bonding is essential in all biological processes involving nucleic acids. After discovering the Watson-crick model of DNA, DNA replication, and Protein synthesis are understood at the molecular level. Watson and Crick found the 3D DNA model consisting of 2 polynucleotide chains twisted around each other in an antiparallel way. The bases are centered in the helix and sugar-phosphate bone at the periphery. The bases such as Adenine and Guanine at one strand, Cytosine, and Thymine on the other strand, making strands complementary to each other.
In replicating DNA, the hydrogen bond between the bases breaks and allows separated strands to make a new hydrogen bond between the bases of newly synthesized strands with DNA polymerase’s help, thus creating a new DNA copy.
DNA replication from the start of replication is two-dimensional. The hydrogen bonding of free DNA nucleotides produces new complementary strains around each parent’s edge as strains begin to open and divide in both directions across the entire DNA molecule.
Importance of hydrogen bonding:
In several biochemical reactions, hydrogen bonding is essential. The unique soluble quality of water is due to hydrogen bonding. Hydrogen bonds are necessary for evaluating the three-dimensional form of folded proteins, including enzymes and antibodies, with complementary DNA strains. Hydrogen bonding stabilizes the DNA double helix structure.
Summary
Hydrogen bonding involves a hydrogen element between partial positive and partial negative atoms. This bond is weaker than a covalent bond but more potent than electrostatic forces.
In DNA, hydrogen bonding plays a significant part in stabilizing DNA structure and configuration. During DNA synthesis and replication, the hydrogen bonds between the bases break and then regenerate to form new DNA.
See Also
Guide to DNA Sequencing
Guide DNA Recombinant Technology